Electrospraying and electrospinning have been used for a wide range of applications ranging from coatings, propulsion, and pesticide sprays to scaffolding a wide range of advanced materials for applications in filtration and tissue engineering. Although electrospun scaffolds have been explored as tissue engineering materials, the scaffolds have not gained traction as they inhibit complete cell infiltration, causing rejection as a foreign body as identified by the immune system.
Both technologies have been demonstrated as having the ability to handle biomolecules without damaging them. John Fenn, PhD, pioneered the use of electrosprays in combination with mass spectrometry to accurately identify molecules by their mass to charge ratio.1 This discovery, widely referred to as electrospray ionization mass spectrometry (ESI-MS), was awarded the Nobel Prize in Chemistry in 2002.
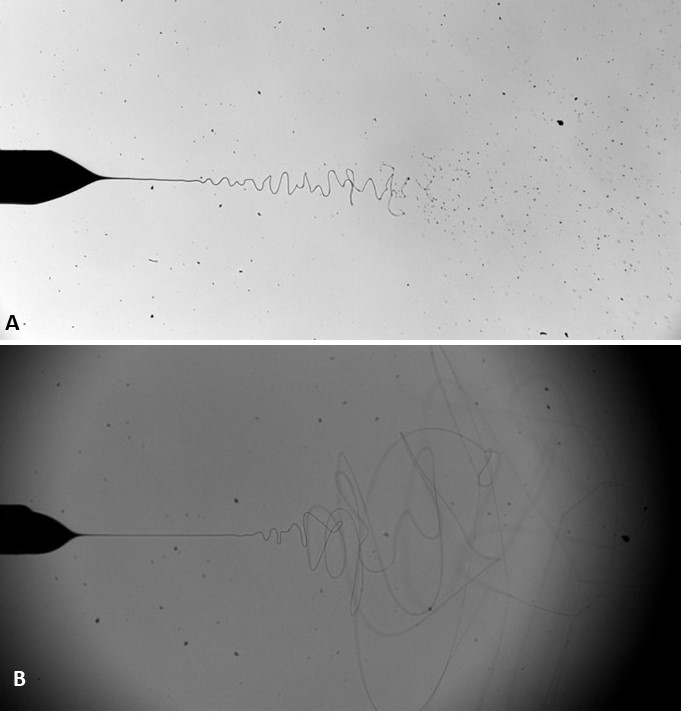
Electrosprays and electrospinning are related, century old, electric field driven technologies. The technologies work on the principal of charging a liquid flowing within a conducting needle, held at a higher potential (usually in the kVs), with respect to a grounded electrode placed centrally, and in line with the needle exit. Liquid properties play a critical role in these technologies, particularly, electrical conductivity, relative permittivity, surface tension, viscosity/rheological properties, and density, which affect stability and other important characteristics. These properties determine whether the process is “electrospraying” or “electrospinning.” In both scenarios the viscosity and surface tension of the liquid are overcome by the electric field, resulting in the liquid being drawn into a cone, and jet.
Other properties of the liquid including the rheological properties determine whether the jet breaks into a stream or a 3D spray plume of droplets or whether the jet elongates into a continuous fiber (Figure 1). Other parameters effecting the process are the flow rate of the liquid supply and the applied voltage to the needle. The applied voltage affects the electric field between the charged needle and the grounded electrode. Although the applied voltage to the needle is in the several kilovolts, usually, the accompanying current is only a few nanoamperes.
Bio-electrosprays and cell electrospinning
In 2005, we explored both electrospraying and electrospinning to assess their ability to directly handle single or multiple cell types, including human and animal immortalized and primary cells, a wide range of stem and induced pluripotent stem cells (iPSCs), and whole fertilized embryos.2,3 Our studies have shown that post-processed cells and embryos are indistinguishable from controls.
Early studies in both these technologies predominantly required a coaxial needle system, as the inner needle would accommodate the cell suspension while the outer needle held the flow of either a low or high viscosity biopolymer having a low electrical conductivity, which shielded the low viscosity and highly conductive cell suspension, giving rise to either stable bio-electrospraying or cell electrospinning, respectively.
Developments in polymer chemistry have given rise to the use of single needle systems, where the cells are directly mixed with the biopolymer and processed. Today, bio-electrospraying and cell-electrospinning are platform biotechnologies for the direct handling of live cells for the reconstruction of living architectures. A wide range of cell bearing architectures have been reconstructed using these technologies ranging from thin/thick, single/multiple cellular beads and sheets (Figure 2) to multi-cellular vessels/arteries which are indistinguishable from native tissues.
These platform technologies have widespread applicability to the health sciences and medicine, in both the laboratory and the clinic. It is exciting to note that these technologies unlike their rival technologies, possess the ability to scale up with ease.
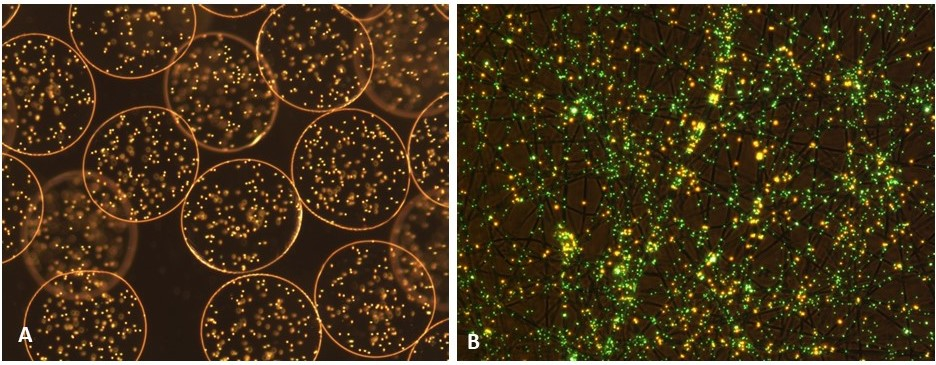
Fully cellular architectures and reconstructs generated using these platforms could allow the storage and preservation of cells and culturing cells in three-dimensions. Reconstructs could be explored for transplantation, repair, replacement, or rejuvenation of damaged and/or aging tissues/organs, the creation and delivery of experimental and/or medicinal cells and/or genes, and in vitro testing/screening of organoids developed through these approaches. Additionally, these technologies have applications in aquaculture, animal welfare and sex selection, and our evolving food industry (from meat free to in vitro meat). The applicability of bio-electrospraying and cell electrospinning to the biomedical and clinical sciences have only just begun. Their versatility will allow their exploration for a wide range of applications.
References
1. Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989(246):64-71.
2. Jayasinghe SN. Bio-electrosprays: from bio-analytics to a generic tool for the health sciences. Analyst. 2011(136):878-890.
3. Jayasinghe SN. Cell electrospinning: a novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst. 2013(138):2215-2223.
Suwan N. Jayasinghe, PhD, is professor of bioengineering at the Centre for Stem Cells and Regenerative Medicine, Institute of Healthcare Engineering and Department of Mechanical Engineering, at the University College London, UK.






